INTRODUCTION
Tuberculosis (TB) is an infectious disease caused by Mycobacterium tuberculosis and spreads through aerosolized droplets [1, 2]. Currently, TB ranks as the second leading cause of death globally due to an infectious agent, surpassed only by coronavirus disease (COVID-19). Annually, TB is responsible for approximately 1.3 million deaths and 7.59 million new cases, affecting an estimated 10.6 million people worldwide, which constitutes nearly one-fourth of the global population. A significant portion of TB cases – approximately 95% – was concentrated in underdeveloped nations, including Indonesia, which ranked as the second most affected country globally [3]. Tuberculosis can be transmitted via various routes and can infect almost any tissue outside the lungs (extrapulmonary TB), accounting for 21.5% of the 33,148 global TB cases, including those affecting ocular tissue [1, 2].
Among patients with pulmonary TB, the prevalence of intraocular TB ranges from 1.4% to 6.8% [4]. The most common ocular manifestations of TB include optic neuritis, neuroretinitis, and posterior uveitis [5]. Hematogenous dissemination of TB from pulmonary or extrapulmonary sites can affect several areas, including the uveal tract, meninges, and choroid [6, 7]. Additionally, neuro-ophthalmic complications, particularly central nervous system TB, are among the most severe forms of the disease. This is evidenced by the high prevalence of TB meningitis, which occurs in approximately 82% of TB meningitis cases and 67% of pediatric tuberculous meningoencephalitis cases, which can present significant morbidity and mortality rates of 30–40% [2, 8, 9]. Diagnosing these conditions is challenging due to nonspecific symptoms and the limited sensitivity of diagnostic tools, making it difficult to confirm cases based solely on clinical and imaging findings [10]. These diagnostic challenges often result in treatment delays and increased mortality rates.
We present a case involving a young adult with unilateral progressive vision loss due to posterior uveitis and optic atrophy, secondary to TB-related meningoencephalitis. These findings underscore the importance of heightened vigilance and consideration of TB as a differential diagnosis in patients presenting with gradual vision loss.
CASE REPORT
A 17-year-old female patient presented with progressive vision loss in her right eye (RE) over the past month. This visual disturbance had gradually worsened and was accompanied by persistent headache, high fever, nausea, and vomiting. Notably, her mother was undergoing treatment for TB. The patient’s body mass index (BMI) was below the 5th percentile, indicating malnutrition.
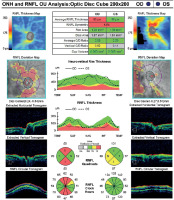
Ophthalmological examination of her best corrected visual acuity (BCVA) in the RE revealed 2/60, diminished pupillary light reaction, 0/38 color vision on the Ishihara test, relative afferent pupillary defect (RAPD), optic disc pallor, and multiple choroidal white lesions (Figure 1A). Examination of her left eye (LE) revealed a BCVA of 6/6, diminished pupillary light reaction, 38/38 color vision on the Ishihara test, segmental disc pallor, and a blurred optic disc margin (Figure 1B). There was no flare or cells in the anterior chamber of either eye, with no additional signs of vitritis or macular edema. Confrontation visual field testing showed a defect in the infratemporal quadrant of the RE. Ocular motility was normal, and cranial nerve examination of the olfactory nerve yielded normal results. Intraocular pressure was normal in both eyes. Slit-lamp examination showed no abnormal findings. Optical coherence tomography (OCT) revealed retinal nerve fiber layer thinning in all quadrants of the RE, while the LE showed nasal and temporal quadrant thinning (Figure 2).
Figure 1
Funduscopic examination revealed optic disc pallor (white arrow) and multiple choroidal white lesions (choroidal tubercles) (red arrows) in the RE (A), and segmental disc pallor and a blurred optic disc margin (white arrow) in the LE (B).
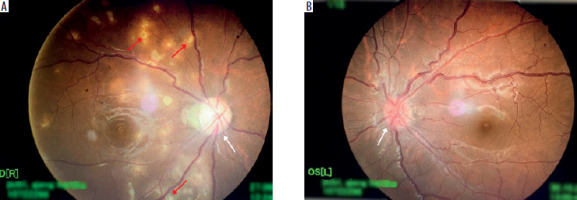
Figure 2
Optical coherence tomography revealed retinal nerve fiber layer thinning in all quadrants of the RE, while the LE showed nasal and temporal quadrant thinning
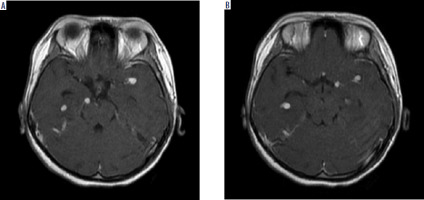
Laboratory investigations revealed elevated D-dimer levels, with other parameters within normal limits. Chest X-ray confirmed a miliary pattern and left pleural effusion, supporting the diagnosis of TB (Figure 3). Cerebrospinal fluid (CSF) analysis showed clear fluid without bacteria or fungi on Gram staining, aerobic cultures, and fungal cultures. The CSF analysis revealed positive Nonne and Pandy tests, elevated total protein levels, and elevated white blood cell (WBC) count with lymphocytic predominance, and low glucose levels, which are indicative of TB meningitis. HIV-1 and syphilis testing were non-reactive. GeneXpert Mycobacterium tuberculosis testing on lumbar CSF was negative. Contrast-enhanced magnetic resonance imaging (MRI) confirmed multiple scattered nodular lesions in the right and left Sylvian fissures, temporal horn lateral ventricles, prepontine cistern, and right fourth ventricle, indicative of tuberculomas. Additionally, multiple lesions in vascular regions suggestive of vasculitis and an encephalomalacial cyst in the right pons were observed (Figure 4).
Figure 3
Chest X-ray demonstrating miliary pattern and left pleural effusion, supporting the diagnosis of tuberculosis.
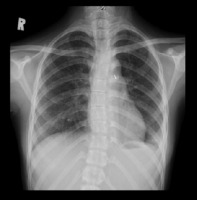
Figure 4
Contrast-enhanced T1-weighted perfusion brain MRI showing multiple scattered nodular lesions in the right and left Sylvian fissures, temporal horn lateral ventricles, prepontine cistern, and right fourth ventricle, indicative of tuberculomas.
The patient was treated with antibiotics and anti-TB medications (ATT), excluding ethambutol, for a 2-month intensive phase followed by a 9-month continuation phase. Oral systemic corticosteroid prednisone was initiated at a dose of 45 mg/day for 2 weeks, then tapered to 30 mg/day. Additional vitamin B6 supplementation was provided. Despite 2 months of treatment, there was no improvement in visual acuity (VA) in the RE, although the number of tubercles in the posterior chamber of both eyes decreased.
DISCUSSION
Ocular TB can affect any part of the eye, including its segments and layers, as well as the optic nerve, leading to significant vision impairment and a reduced quality of life. A range of 1% to 6% represents the prevalence of ocular TB in patients with pulmonary TB [4, 5, 11]. In countries where TB is not widespread, 1% to 6% of individuals with uveitis are affected by ocular TB, while in endemic areas, the prevalence rises to 9% to 11% [11]. In a study involving 64 patients suspected of having TB-related uveitis, 24 (37.5%) reported prior contact with individuals with pulmonary TB, often occurring years before the onset of ocular symptoms [12]. Primary ocular TB, where the ocular structures are the primary site of infection, can present with clinical features such as lid abscess, conjunctival infiltration, scleritis, and interstitial keratitis. Conversely, secondary ocular TB can involve the uveal tissue, retina, and optic nerve, manifesting as a hypersensitivity reaction to systemic TB infections or through hematogenous spread [13].
Intraocular TB can cause permanent vision damage and manifests in various clinical forms, including neuritis, papillitis, solitary or multiple choroidal tubercles, multifocal choroiditis, and choroidal granulomas [5, 12]. Tuberculosis-associated uveitis can be classified into four types – anterior, intermediate, posterior, and panuveitis – with posterior uveitis being the most prevalent presentation [13]. Hematogenous spread of TB from pulmonary or extrapulmonary sites most commonly affects the uveal tract due to its rich vascular supply predisposing it to ocular TB [6, 7, 13, 14]. As a result of localized inflammation brought on by bacilli multiplying within the choroid, choroid tubercles form and have the potential to merge into tuberculomas [7]. Choroidal tubercles, the most common intraocular manifestations of TB, are prevalent in 90% of children and some adults, and infrequently may be associated with pulmonary or extrapulmonary TB, such as tuberculous meningitis and posterior uveitis [5, 15]. In a study involving 100 patients diagnosed with tuberculous meningitis, ocular sequelae were observed in 82% of cases. Among these, 40% exhibited decreased visual acuity, 22% presented with papilloedema, 18% displayed pale discs, and 10% showed choroidal tubercles [16]. In our case where the patient complained of a headache and fever, this complaint is consistent with the symptoms and diagnostic criteria for TB meningitis [9, 17-19]. Patients may experience a relative afferent pupillary defect (RAPD) due to optic nerve injury, as observed in our case in the RE, where the eye with more severe damage exhibited RAPD [8, 17]. Consequently, the patient reported a month-long history of gradual vision loss in the RE; later on, the funduscopic examination revealed bilateral optic atrophy and posterior uveitis. In a TB patient, optic neuropathy can result from various pathophysiological mechanisms, including tuberculomas compressing the optic nerve, toxic optic neuropathy from drugs, chronic papilledema from meningitis, optic neuritis, tubercular perineuritis, elevated intracranial pressure, and arachnoiditis of the optic chiasma. If untreated, optic neuropathy can progress to optic atrophy, eventually leading to irreversible vision loss [17]. The progression of optic atrophy in papilledema can vary significantly, occurring within weeks or days or over months or years, depending on the degree and duration of the raised intracranial pressure [18]. Regular follow-up is essential during the first four to eight weeks to ensure that the edema is resolving [19].
In nearly all documented cases, the diagnosis of intraocular TB was presumptive, compounded by the difficulty in obtaining microbiologic evidence, which makes a definitive diagnosis challenging [5]. Most individuals with ocular involvement do not have a history of pulmonary or other systemic manifestations. Patients with extrapulmonary TB may not exhibit signs of pulmonary TB [20-24]. However, it is crucial to note that negative test results, such as GeneExpert, do not rule out the condition entirely. A presumptive diagnosis of ocular TB can be made based on the presence of at least one clinical sign, combined with positive results from immunological, histopathological, or imaging tests. A family history of TB, especially in endemic regions, along with CSF analysis, chest X-ray, and thorough MRI, can further support the diagnosis and help to avoid treatment delays. Neuroimaging modalities such as MRI are indispensable for excluding intracranial disorders [25]. Classic neuroradiologic features of TB meningitis encompass hydrocephalus, basal meningeal enlargement, cerebral edema, nodular enhancing lesions, and hypodensities indicative of cerebral infarcts may be discerned [9]. In the brainstem and spinal area, MRI is more recommended than computed tomography for identifying specific anomalies associated with progressive vision loss [9, 13, 26].
Antituberculous therapy (ATT) is the cornerstone of treatment for TB uveitis and TB meningitis, akin to systemic TB, and aims to reduce morbidity and mortality rates. The standard four-drug regimen comprises isoniazid, rifampin, pyrazinamide, and either streptomycin or ethambutol, administered daily during the 2-month intensive phase, with the potential for extension up to a 9-month period [9, 13, 26, 27]. However, there is still uncertainty regarding the best course of action for those suffering from tuberculous optic neuropathy. Due to the higher risk of toxic neuritis or ocular neuropathy in these patients, ethambutol should be avoided. Two important risk factors for subclinical toxicity have been identified: the duration of drug administration and ethambutol-induced visual neuropathy [17, 28]. Research indicates that when systemic corticosteroids and ATT are administered simultaneously to individuals suspected of having tuberculous uveitis, the outcome is favorable [5, 12]. To control the concomitant inflammatory response and reduce macular edema, oral prednisone can be administered in the treatment of ocular TB [12]. Similar to other studies, our report found that VA did not improve after 2 months of initial treatment [29, 30]. By contrast, ATT therapy has been shown to improve VA in a number of cases, after 14 days [31], 1 month [7], or 9-10 months [13, 18], with or without choroidal tubercle shrinkage [7, 18, 25, 31].
This case underscores the importance of heightened awareness of TB and its extrapulmonary manifestations, particularly when a close contact has the disease. It also highlights the necessity of a comprehensive diagnostic approach when evaluating patients with progressive visual loss in endemic regions. Auxiliary investigations, such as neuroimaging, are indispensable for promptly initiating targeted therapy, which ultimately culminates in enhanced patient outcomes and quality of life. Further research and advancements will continue to enhance our comprehension of the complexity of clinical presentations and the significance of concomitant pathologies in TB infection.

 ENGLISH
ENGLISH




