INTRODUCTION
The cornea is a critical component of the eye’s optical system, providing approximately 43.25 D of optical power, which accounts for about two-thirds of the total optical power of the eye. The structure of the cornea and any associated diseases directly impact its function, which is crucial for the vision process [1-3]. Another important component of the optical system is the transparent lens, which has a power of approximately 19.0 D. The lens adjusts its refractive power during accommodation, enabling sharp vision for both distant and near objects. However, with age, the ability of the lens to accommodate gradually decreases and eventually ceases as the lens hardens. The remaining components of the ocular optical system include the aqueous humor and the vitreous body. While these structures contribute to the eye’s refractive power, their impact is relatively minor and does not significantly influence the overall optical processes. Corneal disorders often present with symptoms such as blepharospasm, lacrimation, photophobia, reduced visual acuity, and conjunctival hyperemia. The cornea is protected by a tear film, which maintains moisture on the surface of the cornea and bulbar conjunctiva. Tear deficiencies – whether congenital or acquired – have an adverse impact on the cornea, leading to dryness, symptoms of dry eye syndrome, and disruption of light transmission into the eye. The cornea is nourished by tears, perilimbal blood vessels, and aqueous humor, and it is innervated by a ranch of the sixth cranial nerve. The cornea is demarcated by the limbus. Its shape resembles a spherical segment, with a curvature similar to watch glass. The axial length of the eye (measured from the corneal apex to the posterior pole) increases significantly from infancy until 14 years of age, reaching 22.9 mm [2]. The axial length of the eye in adults is 23-24 mm, while the cornea measures 12 mm horizontally and 11 mm vertically. Corneal thickness is not uniform: in the central part, it is approximately 0.6 mm, while at the periphery it increases to about 1.0 mm. The central part of the cornea, spanning a diameter of 4 mm, serves an optical function [4]. A healthy cornea is avascular, and comprises both cellular and acellular elements. The cellular components include epithelial cells, keratocytes, and endothelial cells. Epithelial cells originate from the epidermal ectoderm. Keratocytes and endothelial cells are derived from the neural crest [3]. The acellular components consist of collagen and glycosaminoglycans.
The optical properties and refractive capabilities are determined by the histological structure of the cornea [5]. Until recently, it was believed that the cornea consists of five fundamental layers: the epithelium, which remains in contact with the conjunctival epithelium and is renewable; the anterior limiting lamina – Bowman’s membrane, composed of collagen fibers; the corneal stroma (substantia propria), formed of fibrous connective tissue, with keratocytes and leukocytes present in its structure; the posterior limiting lamina – Descemet’s membrane, resistant to injury, with regenerative capacity; the inner corneal layer constituting a single-layered, flat endothelium consisting of hexagonal cells tightly adherent to one another; the endothelium remains in contact with the aqueous humor, the endothelium lacks regenerative capacity. Recent studies indicate the existence of an additional layer of corneal cells, named (after its discoverer) the Dua’s layer [6], located anterior to the Descemet’s membrane. Dua’s layer plays a role in organizing the collagen fiber meshwork in the drainage angle, contributes to the outflow of aqueous humor, and helps maintain normal intraocular pressure. Dua’s layer is tough and exhibits resistance to external factors. The cellular structure of the cornea ensures its transparency and supports its essential functions within the optical system.
Normal endothelial cell density ranges from approximately 2,500 to 3,000 cells/mm2. When corneal cell density drops below 500 cells/mm2, there is a significant risk of the cornea losing its optical power. A reduced endothelial cell count compromises the integrity of this layer, allowing aqueous humor to penetrate the corneal stroma. This disrupts the physiology of endothelial cells, leading to corneal edema and contributing to opacification, along with symptoms of vesicular degeneration. As previously mentioned, the corneal endothelium lacks the capacity for spontaneous regeneration. Defects in the endothelial cell layer are compensated by proliferation and the enlargement of existing endothelial cells, which penetrate between the expanding normal cells of this corneal layer. A decrease in endothelial cell count and their abnormal morphology impair normal corneal function.
The process of vision is a multifaceted, multi-stage cycle involving various types of changes. Correct vision depends not only on the health of the visual organ, including the cornea, but also on broader factors such as the child’s physical growth, mental predispositions, living conditions, intellectual capabilities, and interactions with their surroundings. These abnormalities can lead to difficulties in school learning and other educational processes and, in the future, hinder the ability to pursue professions requiring accurate stereoscopic and color vision, and, importantly, make everyday communication with the environment challenging.
Light rays passing through the intact cornea, pupil, and other normal optical structures of the eye are focused on the retina, where they are detected by the light-sensitive layers of photoreceptor cells, including cones and rods. The number of cones is approximately 6 million, and they are primarily concentrated in the central retina, particularly in the macular and foveal regions. Cones are responsible for photopic (daylight) vision and color perception. There are three types of cones, responsible for distinguishing primary colors. Rods, numbering approximately 120 million, facilitate low-light vision and night vision (scotopic vision), and are predominantly located in the peripheral retina. The light-sensitive components of the retina convert light stimuli into neural impulses through complex biochemical and neurochemical processes. This initiates subsequent visual processes involving the optic nerves, which transmit visual signals to the central nervous system and the visual centers in the brain’s occipital lobe. In the cortical visual centers, these impulses are received, processed, and transmitted to other brain regions, allowing for the assimilation of visual information and the interpretation of images of the external world. Newborns have basic light perception, distinguish between light and dark, and respond to light stimuli by squinting. In the following months and years, the child’s visual development improves, eventually reaching its mature form under normal conditions. This mature vision is characterized by simultaneous binocular (stereoscopic) vision and proper color recognition. Stereoscopic vision (stereopsis, or depth perception) is responsible for the ability to perceive depth and accurately assess the distance between objects. In healthy eyes, a slight asymmetry in the alignment of the eyeballs during binocular vision allows the brain to merge the two images. This fusion creates a three-dimensional perception of the surrounding world. Anatomical and/or functional disorders of the visual organ, including the cornea, hinder or entirely obstruct the maturation of this sensory system, adversely impacting the child’s visual development.
The organ of vision processes over 80% of the information received from the outside world. In children, its structure and function are not yet fully developed and differ from the size and functionality of this sensory organ in adults. Following intrauterine development, the eyes continue to grow and refine their function systematically from infancy through to adulthood. Their development occurs in two distinct phases. The eye undergoes rapid growth from birth to two years of age, followed by a slower growth phase between two and 14 years of age [2]. The growth of the eye and the significant development of vision occur during early childhood – in infancy and the preschool years – as the child’s interest in the environment increases and the central nervous system matures. The further growth and development of the eye proceed at a slower pace, with its final size and functionality typically reached by the age of 18, or, in some cases, slightly later, around the age of 21. Any anatomical or functional disorders affecting the visual organ can hinder or prevent the proper development of vision in a child. The physicochemical properties of the cornea enable the transmission of light rays from the external environment within the optical window. The optical window represents the segment of the (electromagnetic) light spectrum that passes through Earth’s atmosphere, ranging from 380 nm to 780 nm, and is perceptible to humans. Wavelengths above 780 nm fall into the infrared range, which is invisible to the human eye, while wavelengths below 380 nm belong to the ultraviolet spectrum, also inaccessible to human vision. An important element of the vision process is visual acuity, which refers to the ability to distinguish fine details. For the eye to distinguish two objects as separate, the light rays must stimulate two different cones, with one unstimulated cone between them. The reference measure for visual acuity is the ability to discern two distinct points separated by an angle of one minute of arc from a distance of 5 m, or 10 s of arc from a distance of 10 m. Uncorrected refractive errors can disrupt the development of proper vision in children, leading to reduced visual acuity and potentially causing amblyopia.
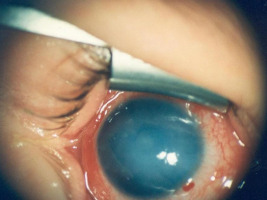
The normal cornea is transparent, smooth, and glossy. These qualities are ensured by its unique anatomical structure. Congenital abnormalities and acquired corneal diseases can hinder proper visual development in children. One of the congenital causes of corneal changes is congenital nasolacrimal duct obstruction, often noticed early by caregivers. Prolonged lacrimal duct obstruction can lead to the accumulation of mucous and/or purulent discharge on the corneal surface, causing keratitis and altering the optical power of the anterior corneal surface due to discharge coating, resulting in refractive error (hyperopia). Unilateral lacrimal duct obstruction can contribute to the development of monocular hyperopia with secondary amblyopia. In cases of bilateral obstruction, it may also lead to refractive changes in both eyes. Other causes of congenital corneal changes include abnormalities in the visual system associated with premature birth. In some premature infants, corneal edema may occur alongside retinal changes, reducing corneal transparency. These changes typically regress as the visual system matures (Figure 1).
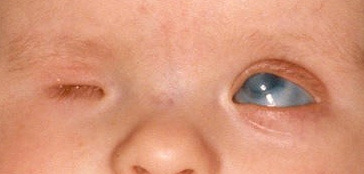
Corneal edema with loss of transparency also occurs in congenital glaucoma (Figure 2). The diagnosis of glaucoma is confirmed by other characteristic ocular changes.
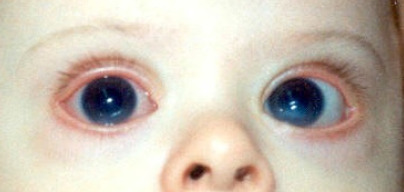
Sclerocornea is a congenital condition characterized by abnormalities in the sclera, which is thin and deformed, as well as in the cornea, where opacities of varying intensity and size are present. Sclerocornea refers to the lack of a clear demarcation between the cornea and sclera. Corneal changes may lead to a marked decrease in visual acuity (Figure 3).
Euryblepharon, characterized by an increased width of the palpebral fissure disproportionate to its vertical dimension, may present as an isolated condition or in association with other ocular or systemic abnormalities [7] (Figure 4). In severe cases, it can lead to hare eye (lagophthalmos) and keratopathy. This developmental abnormality is rare; it was observed by the authors of this article in a single pediatric case.
Keratoconus is another condition that may develop in childhood and progress over time. Keratoconus has a genetic predisposition and is also associated with allergic eye diseases and the use of ill-fitting contact lenses. Additionally, frequent eye rubbing has been linked to the development of the condition. Keratoconus involves corneal thinning and its protrusion, which may occur centrally or paracentrally. As the disease progresses, corneal deformation becomes more pronounced, resulting in reduced visual acuity, increased light sensitivity, astigmatic refractive error, blurred vision, and/or diplopia.
Polish ophthalmologist Bazyli Bogorodzki was the first physician in the country to comprehensively address the issues associated with this abnormality in the 1980s, based on his own research [8]. Developmental disorders such as megalocornea also alter its role as a factor influencing vision. Mega-locornea is characterized by increased corneal dimensions, with a diameter of at least 13.0 mm. The enlarged corneal size induces refractive errors, typically resulting in myopia ranging from –5.0 D to –7.0 D, which requires optical correction. A normal endothelial cell layer in megalocornea does not impair visual function. Megalocornea must be differentiated from buphthalmos and high myopia. The opposite of megalocornea is microcornea, defined by a corneal diameter of less than 11 mm. It may coexist with microphthalmos, leads to refractive errors, and causes hyperopia – typically high-grade – requiring optical correction. Another developmental defect of the cornea is keratoglobus, characterized by generalized thinning across the entire corneal surface, giving it a spherical appearance. In keratoglobus, the anterior stromal layer is absent, with visible ruptures in Descemet’s membrane, while the corneal endothelium remains unchanged. To evaluate corneal lesions in cases of congenital corneal staphyloma, ultrasound examination of the eye is a valuable diagnostic tool [9]. Genetically determined metabolic disorders can also lead to corneal opacities, necessitating proper diagnosis of the underlying cause to initiate an appropriate treatment. Congenital changes, dystrophic corneal structures of genetic origin, as well as acquired degenerative corneal changes – post-inflammatory in nature – also adversely affect visual processes, may distort the cornea, and lead to loss of its transparency. Corneal dystrophy refers to a heterogeneous group of bilateral, genetically determined, non-inflammatory corneal disorders. These conditions are confined to the cornea and are not linked to systemic inflammation or symptoms of other etiologies [9, 10]. Corneal degenerations occur as a secondary consequence of prior corneal disease and are often referred to as ocular ‘fingerprints’ of previous damage to this part of the eye. These changes can be either unilateral or bilateral. A hallmark of corneal degenerative changes is the presence of white or yellowish stromal deposits composed of cholesterol, fats, and phospholipids. Dystrophic and degenerative changes significantly impair visual processes and can even lead to their complete loss.
An etiological factor in acquired corneal pathological conditions is vitamin A deficiency. As a component of the retinal pigment, vitamin A plays a critical role in the perception of visual stimuli, and its deficiency can impair this process. Other manifestations of acquired corneal disorders include ocular infections of various etiologies (bacterial, fungal, and viral), including herpes simplex virus keratitis [11-15]. Foreign bodies in the cornea, chemical damage to the eye, and mechanical injuries to the cornea – conditions that can also occur in children – also cause corneal diseases that negatively affect the visual process. Corneal injuries, often contaminated with plant debris, account for over 60% of trauma-related cases of fungal etiology [14, 15]. Prolonged wearing of contact lenses, especially without their removal at night, improper disinfection, poor hygiene during storage, and long-term use of the same lenses, can also be etiological factors in corneal inflammation.
Pathological changes in the cornea in most children are associated with a loss of transparency, partial or complete opacification, corneal distortion, and/or refractive errors. Central opacities are particularly detrimental to the development of vision in children, as they obstruct the proper passage of light through the optical pathway into the eye, preventing it from reaching the photosensitive layer of the retina. This often causes the forced positioning of the head and eyes to see the observed object, reduces visual acuity, and results in the development of amblyopia and strabismus.
The cornea also plays another crucial role in the visual system – it serves as a protective barrier and outer layer of the eyeball, forming its anterior part. The protective function of the cornea is also associated with its dense sensory innervation. Notably, the cornea is 100 times more sensitive than the conjunctiva [5]. At the same time, the unique anatomical features of cellular structure and physiological properties account for the cornea’s optical nature and refractive characteristics.
The diagnosis of corneal disorders begins with a detailed medical history and an ophthalmologic examination, adapted to the child’s age and level of cooperation with the examiner. Establishing a good relationship between the ophthalmologist and the child’s parents is also important. In young, non-cooperative patients, diagnostic procedures are performed under general anesthesia. For older cooperative children, additional diagnostic tests can be performed without general anesthesia to support the diagnosis. These include tonometric, perimetric, and pachymetric examinations, as well as imaging diagnostics: ocular ultrasound, tomographic assessment, and magnetic resonance imaging. Of particular importance is the evaluation of corneal structure using ultrasound biomicroscopy [16]. Genetic testing should also be considered in the differential diagnosis, along with consultative examinations.
Treatment, depending on the etiology, may be causal, symptomatic, or a combination of both. In many corneal conditions, early diagnosis and therapy can eliminate the underlying causes and help preserve the physiological characteristics of the cornea. Causal treatment includes conservative management and pharmacological therapy. Depending on the indications, surgical treatment is also implemented, including corneal transplantation, after the inflammatory process of the disease has been stabilized. One of the initial approaches to treatment, when feasible, involves correcting refractive errors with the use of spectacle lenses or contact lenses. Also, recently, there has been increased emphasis on the role of proper nutrition in supporting the development of the visual system in children, particularly those born prematurely [17]. Research has demonstrated that nutrition plays a pivotal role in these processes. Infant formulas containing nutrients essential for healthy visual development – particularly omega-3 fatty acid docosahexaenoic acid (DHA) and omega-6 fatty acid arachidonic acid (AA) – may protect non-breastfed infants from abnormalities in the development of this sensory organ. Children undergoing ophthalmic treatment for corneal diseases require regular follow-up examinations.
Changes in the visual organ of newborns, infants, and young children observed by parents or guardians warrant an urgent ophthalmological examination to evaluate the condition of the eyes and initiate appropriate interventions based on the identified abnormalities, utilizing available modern diagnostic and therapeutic methods.

 POLSKI
POLSKI