INTRODUCTION
Gene therapy using voretigene neparvovec (Luxturna) represents a breakthrough in the treatment of inherited retinal dystrophies (IRD) associated with biallelic mutations in the RPE65 gene. Voretigene neparvovec is a medication based on an adeno-associated virus type 2 (AAV2) vector, which delivers a functional copy of the human RPE65 gene directly to the retinal pigment epithelium (RPE) cells [1]. The expression of the gene results in the production of functional RPE65 protein, which is essential for the proper course of the visual cycle [2]. Clinical studies have shown that a single subretinal administration of voretigene neparvovec can lead to improvement in vision, including patients’ ability to orient themselves in low-light conditions, which translates into a significant enhancement in their quality of life [3]. In December 2017, the U.S. Food and Drug Administration (FDA) approved voretigene neparvovec as the first gene therapy for an inherited retinal disease, marking a milestone in the advancement of gene therapies in ophthalmology [4]. The drug was approved for marketing in Europe in 2018. In 2021, our Poznan team performed the first subretinal administration of the product in Poland.
Voretigene neparvovec is indicated for the treatment of patients with confirmed biallelic RPE65 mutations and sufficient viable retinal cells, regardless of the clinical phenotype [1]. Biallelic mutations in the RPE65 gene are responsible for a spectrum of inherited retinal dystrophies referred to as RPE65 mutation-associated inherited retinal dystrophies (IRDs). They include Leber congenital amaurosis type 2 (LCA2), sometimes referred to as early-onset severe retinal dystrophy (EOSRD), as well as retinitis pigmentosa type 20. It is estimated that mutations in the RPE65 gene account for 1–3% of LCA cases [1, 2] and 0.6–6% of RP cases [3]. The majority of patients present with severe visual impairment, significant deterioration of twilight vision, nystagmus, and markedly reduced retinal activity on electroretinography (ERG).
The aim of this study is to provide a preliminary assessment of the efficacy and safety of gene therapy with Luxturna in the first four Polish patients with biallelic mutations in the RPE65 gene, with particular emphasis on the therapy’s impact on visual acuity, visual field, and mobility performance under varying luminance levels (as measured by the Multi-Luminance Mobility Test, MLMT).
MATERIAL AND METHODS
Patient eligibility assessment
A total of four patients (n = 4 eyes) with genetically confirmed biallelic RPE65 mutations met the eligibility criteria for Luxturna treatment. Mutation confirmation was based on genetic testing results, including next-generation sequencing (NGS) of a panel of genes associated with inherited retinal dystrophies (n = 3), or (in the case of family examinations), sequencing of selected RPE65 gene amplicons (n = 1). To be eligible for treatment, patients had to be at least 3 years old and demonstrate sufficient viable retinal cells, as confirmed by imaging assessments, including optical coherence tomography (OCT) and fundus autofluorescence (FAF). In OCT examinations, the central foveal thickness had to be at least 100 µm [4, 5] to be considered acceptable. Fundus examination required a non-atrophic area in the macula measuring at least three optic disc diameters. Additionally, the visual field, as assessed by Goldmann perimetry (ZEISS Humphrey Field Analyzer 3, Carl Zeiss Meditec AG, Germany), had to be preserved within 30° of the fixation point. Patients with advanced retinal atrophy, no light perception, or other ocular conditions that could affect the assessment of the therapy’s efficacy and safety were excluded from treatment. All procedures were performed in accordance with the manufacturer’s recommendations and the guidelines outlined in the drug’s Summary of Product Characteristics (SmPC). Due to the lack of treatment reimbursement, patients received the medication in only one eye. In three cases, it was the worse-seeing eye, while in one case, it was the better-seeing eye. The decision was made after discussion with the patient and, in the case of minors, with their guardians.
Subretinal administration of gene therapy
For 3 days before the procedure, patients received immunomodulatory treatment (prednisone at a dose of 1 mg/kg body weight per day) according to the regimen specified in the drug’s SmPC. The administration of voretigene neparvovec was performed in an operating room setting, under local anesthesia (n = 2) or general anesthesia (n = 2). The procedure was performed by a team of experienced vitreoretinal surgeons (M.S. and P.R.) via pars plana approach, using a standard 25G three-port vitrectomy (Constellation Vision System, Alcon, USA). During the procedure, a triamcinolone suspension was administered to visualize the vitreous body. In the next step, 0.3 ml of Luxturna suspension – containing 1.5 × 10¹¹ vector genome (vg) particles of voretigene neparvovec – was administered into the subretinal space using a 41G cannula connected to a 1 ml syringe, creating a bleb encompassing the macula. In one case, it was necessary to create two blebs. During the procedure, an intraoperative OCT system (RESCAN 700, Zeiss, Germany) was used to monitor the location of the cannula and to ensure precise drug delivery in two patients (n = 2). After drug administration, a fluid–air exchange was performed. Following surgery, patients were instructed to remain in a supine position for 24 hours. Standard postoperative eye drops, including a steroid and an antibiotic, were administered, and immunomodulatory treatment was gradually discontinued.
Clinical evaluation of patients
Before and after treatment, comprehensive ophthalmic examinations were performed, including assessment of best-corrected visual acuity (BCVA), color vision, measurement of intraocular pressure (IOP), slit-lamp examination, fundus examination, spectral-domain optical coherence tomography (SD-OCT), and FAF. Additionally, visual field testing was performed using Goldmann perimetry. To document the appearance of the fundus, ultra-widefield fundus photographs (California®, Optos Inc, Marlborough, USA) were taken before and after the procedure. Patients’ functioning under varying lighting conditions was assessed using the mobility test (MLMT), conducted at luminance levels of 1, 4, 10, 50, 100, and 400 lux. All patients underwent electrophysiological testing in accordance with ISCEV (International Society for Clinical Electrophysiology of Vision) standards. Postoperative clinical assessments were conducted for all patients on days 1 and 7 after injection; at 1 and 3 months (n = 4 eyes) and 6 months after the procedure (n = 3 eyes); and subsequently at 12 months (n = 2 eyes), 18 months (n = 2 eyes), and 42 months (n = 1 eye). In some cases, examinations were performed more frequently between intervals.
RESULTS
Demographic and genetic data of the patients
Four patients underwent treatment: two diagnosed with LCA2 (P1, P4) and two with RP20 (P2, P3). All patients had confirmed biallelic mutations in the RPE65 gene. In three cases, the mutations were present in a compound heterozygous state, while in one patient they were homozygous (Table I).
Table I
Patient demographics and mutation type characteristics
Preoperative characteristics of the patient group
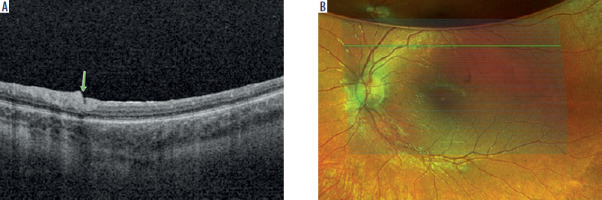
All patients presented with decreased visual acuity and impaired twilight vision from early childhood. Nystagmus was also clinically present in two patients. Visual acuity ranged from 3.7 (light perception) to 0.30 logMAR (0.50). Fundus examination revealed atrophic changes and defects in the RPE layer in the macula, along with vascular narrowing. OCT imaging revealed thinning of the outer retinal layers accompanied by increased signal penetration into the choroid (Figures 1 and 2). The central foveal retinal thickness measured at least 100 µm, with values ranging from 122 to 166 µm. Detailed clinical data are provided in Table II.
Figure 1
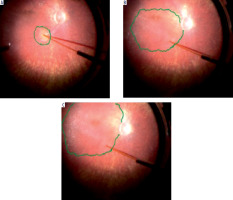
Fundus photograph of the right eye and horizontal 12-mm OCT scan of patient P2 with RP phenotype prior to gene therapy administration.
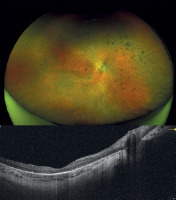
Figure 2
Fundus photograph of the right eye and horizontal 12-mm OCT scan of patient P4 with LCA phenotype prior to gene therapy administration.
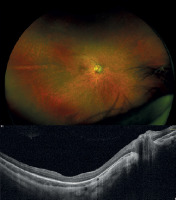
Table II
Clinical characteristics of patients (P1, P2, P3, P4) before gene therapy administration. Visual acuity of the treated eye is indicated in bold.
Subretinal administration of voretigene neparvovec
In the presented case series, the procedure of subretinal administration of voretigene neparvovec was completed without intraoperative complications in all four patients. In each case, a bleb covering the macular area was successfully created (Figure 3), which is crucial for the therapy’s effectiveness since this region contains the highest density of photoreceptors. In patient P4, it was necessary to create more than one bleb to cover the entire targeted subretinal space. To facilitate precise drug delivery, an intraoperative OCT system was additionally used in two cases (P3 and P4) to monitor the position of the cannula and confirm the correct administration of the drug into the subretinal space. The use of these techniques, consistent with the latest reports [6], minimized the risk of retinal damage and improper vector distribution.
Postoperative course and adverse effects
No complications such as hemorrhage, secondary glaucoma, or retinal detachment were observed during the postoperative follow-up. In all patients, the subretinal bleb was absorbed within 24 hours. Air remained in the eye for 7–10 days. Mild conjunctival hyperemia, typical after vitrectomy surgery, was observed in all patients and resolved after 4 weeks of standard treatment with steroid eye drops. At the site of 41G needle insertion, disruptions in retinal architecture were observed; however, retinal continuity was preserved (Figure 4).
Effectiveness of gene therapy
The follow-up period varied across the study group, lasting 42 months for P1, 18 months for P2, 6 months for P3, and 3 months for P4.
Visual acuity
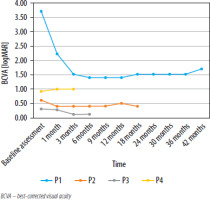
During the 42-month follow-up period of patient P1 with LCA, an improvement in visual acuity was initially observed. Prior to gene therapy, the patient had only light perception. After 6 months, visual acuity improved to 1.40 logMAR (0.04). However, 18 months after Luxturna administration, a gradual decline in improvement was observed, with BCVA reaching 1.52 logMAR (0.03), then further decreasing to 1.70 logMAR (0.025) at 42 months (Table III). After 30 months, no light perception was found in the eye that did not receive gene therapy (the right eye). It can be assumed that light perception would have also been lost in the left eye if the patient had not undergone treatment.
Table III
BCVA and the difference from the baseline in patient P1 on the logMAR scale (negative values indicate improvement in visual acuity)
During the 15-month observation of patient P2 with RP (Figure 1), who had a baseline visual acuity of 0.60 logMAR (0.25) in the right eye and 0.40 logMAR (0.40) in the left eye, an improvement in visual acuity in the treated right eye to 0.40 logMAR (0.40) was noted one month after gene therapy. Visual acuity in the right eye ranged between 0.40 and 0.50 logMAR (0.40–0.32) over the following 15 months (Table IV). In the untreated left eye, visual acuity increased to 0.20–0.30 logMAR (0.63–0.50) but returned to baseline after 15 months.
Table IV
BCVA and the difference from baseline for patient P2 (in logMAR units). Negative values indicate an improvement in visual acuity.
| Time | BCVA (RE) (logMAR) | Difference from baseline (logMAR) |
|---|---|---|
| Baseline assessment | 0.60 | |
| 1 month | 0.40 | –0.20 |
| 3 months | 0.40 | –0.20 |
| 6 months | 0.40 | –0.20 |
| 9 months | 0.40 | –0.20 |
| 12 months | 0.50 | –0.10 |
| 15 months | 0.40 | –0.20 |
In patient P3 with RP, the baseline visual acuity was 0.30 logMAR (0.50) in the eye treated with gene therapy and 0.40 logMAR (0.40) in the fellow eye. During the 3-month follow-up, a significant improvement in visual acuity was observed, reaching 0.12 logMAR (0.76) in the right eye treated with gene therapy (Table V), while binocular visual acuity improved to 0.08 logMAR (0,86). Similar to patient P2, an improvement in visual acuity was also noted in the untreated (left) eye, reaching 0.22 logMAR (0.56) at 6 months.
Table V
BCVA and the difference from baseline for patient P3 (in logMAR units). Negative values indicate an improvement in visual acuity.
| Time | BCVA (RE) (logMAR) | Difference from baseline (logMAR) |
|---|---|---|
| Baseline assessment | 0.30 | |
| 1 month | 0.28 | –0.02 |
| 3 months | 0.12 | –0.18 |
| 6 miesięcy | 0.12 | –0.18 |
During the 3-month follow-up of patient P4 with LCA (Figure 2), who had a baseline visual acuity of 0.92 logMAR (0.12) in the right eye and 1.22 logMAR (0.06) in the left eye, a slight decline in visual acuity was observed in the right eye treated with gene therapy (Table VI) – to 1.0 logMAR in the right eye (0.10) and to 1.3 logMAR in the fellow (left) eye (0.05). Binocular visual acuity remained at a similar level.
Table VI
BCVA and the difference from baseline for patient P4 (in logMAR units). Positive values indicate a deterioration in visual acuity.
| Time | BCVA (RE) (logMAR) | Difference from baseline (logMAR) |
|---|---|---|
| Baseline assessment | 0.92 | |
| 1 month | 1.00 | 0.08 |
| 3 months | 1.00 | 0.08 |
Changes in BCVA [logMAR] at subsequent time points for the eyes treated with gene therapy are presented in Figure 5.
Visual field
For visual field (VF) testing conducted using kinetic perimetry with the III4e stimulus, the outcome measure was the sum of all degrees in 24 meridians counted at 15-degree intervals. A higher value indicated a greater visual field and a larger area of functional retina.
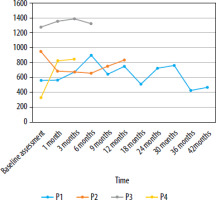
In patient P1, the highest visual field value, measured using kinetic perimetry III4e, peaked at 6 months, representing a 60.47% increase from baseline. After reaching its maximum value, a gradual decline was observed, particularly pronounced between months 6 and 9, and between months 30 and 36.
During the follow-up of patient P2, a decline in visual field parameters was noted in the first month after gene therapy administration, persisting until month 6. The best results were recorded prior to the initiation of treatment, while the worst occurred at 15 months. Between months 6 and 12, an improvement in the visual field was observed; however, this was only a temporary trend, followed by a subsequent deterioration. During the 3-month follow-up period of patient P3, visual field values in the eye treated with gene therapy steadily increased. The highest value was recorded at 3 months, representing an 8.46% increase relative to the baseline value. A 6-month follow-up showed a slight decline. In patient P4, a significant improvement in visual field parameters was observed in the gene therapy-treated eye during the 3-month follow-up period. After the first month, the increase was 147.6%, while at 3 months it reached 152.69% compared to the baseline.
An analysis of four cases indicates that patient P4 experienced the greatest improvement in visual field, with an increase of 152.69%. Patient P3 demonstrated the most stable improvement. In patients P1 and P2, long-term improvement was observed; however, the rate of change varied. Changes in kinetic visual field (III4e) parameter values for patients P1–P4 at sequential time points in the gene therapy-treated eyes are shown in Figure 6.
Multi-Luminance Mobility Test
Analysis of the MLMT results demonstrated an improvement in patients’ navigational abilities after gene therapy. Patient P1, who failed all test levels pre-treatment, post-therapy passed the test at 400 lux (left eye) and 250/400 lux (binocular). Patient P2 showed no significant improvement, still passing only at 4 lux (right eye) and 1 lux (binocular). Patient P3, previously passing at 1 lux, demonstrated improved test accuracy and completion time. Patient P4 pre-therapy passed at 250 lux (monocular) and 50 lux (binocular). Post-treatment, the patient achieved 1 lux performance both monocularly and binocularly, indicating 5-level and 3-level improvements, respectively. Detailed MLMT results for this Polish patient cohort were published by our team separately in “Klinika Oczna” [“Acta Ophthalmologica Polonica”].
DISCUSSION
This study presents the outcomes of treatment with voretigene neparvovec in the first four Polish patients with inherited retinal dystrophies caused by biallelic mutations in the RPE65 gene. Due to the unavailability of publicly funded therapy, treatment was limited to only one eye. One of the key parameters was the evaluation of changes in visual acuity following therapy.
Analysis of the four cases reveals that the most significant therapeutic effect was achieved in patient P3 with RP, who had a marked improvement of at least 0.30 logMAR – a widely recognized clinically significant threshold, corresponding to a gain of three or more lines on the ETDRS chart [7]. Patient P1 showed clear improvement; however, after 18 months, the effect diminished. Nevertheless, the treatment prevented complete vision loss, which occurred in the fellow eye. Patient P2 exhibited an initial improvement, followed by stabilization and slight fluctuations in visual acuity. In patient P4, a minimal decline in visual acuity was observed in the eye treated with gene therapy; however, this patient had the shortest follow-up period (3 months). In three patients, the drug was administered to the eye with poorer visual acuity, and in one patient, to the eye with better visual acuity. In cases where the therapy was administered to the worse-seeing eye, the eye that was initially weaker achieved visual acuity equal to or better than that of the fellow eye following gene therapy.
The results of visual acuity after Luxturna therapy are varied, and not all patients experience improvement. It can be assumed that visual acuity may not be the primary measure of therapeutic efficacy, as the drug mainly affects rod cell activity. Nevertheless, stable BCVA or a slight improvement was observed in most eyes. Factors that may influence outcomes include age, baseline visual acuity, and the presence of other ocular conditions. Some studies have shown a tendency for greater improvement in visual acuity in pediatric patients compared to adults. Russel et al. reported an average improvement of 7.4 letters a year after therapy [8]. In another study, during a 4-year follow-up, Maguire et al. observed stabilization of visual acuity in patients after gene therapy [9]. Stabilization was considered a beneficial outcome, as the natural course of the disease typically involves a decline in vision over such a long period. Lorenz et al. [10] reported significant visual acuity deterioration (≥ 0.3 logMAR) in 5 of 18 treated adult eyes, with significant improvement (≥ 0.3 logMAR) noted in 2 of 18 eyes. The same investigators observed significant visual acuity gains in 2 of 8 pediatric eyes. In the group of patients described by Deng, the mean visual acuity improved from 0.98 logMAR at the onset of the study to 0.83 logMAR at the final follow-up visit [11]. In this study, treatment with Luxturna was associated with a reduction in nystagmus amplitude in children. According to the authors, reduced nystagmus may also contribute to improved visual acuity, as the eyes are better able to focus on observed objects.
Another key goal of gene therapy with voretigene neparvovec, besides improving visual acuity, is to enhance visual field parameters, which are essential for patients’ daily functioning, mobility, and spatial orientation. In the presented group of patients, all showed improvement in visual field parameters as assessed by Goldmann perimetry. These findings align with the results of a phase 3 clinical study demonstrating statistically significant improvements in visual field parameters assessed by Goldmann perimetry at the one-year follow-up after voretigene neparvovec treatment [8]. Similar observations were also made by Maguire et al. [9], who reported an increase in the sum of total degrees using the Goldmann III4e stimulus. This indicates an enlargement of the area of retinal sensitivity and a potential improvement in peripheral vision. In the group of participants who received the drug, this improvement was also maintained after two years.
For patients with RPE65 mutations presenting with impaired twilight and night vision, a key efficacy measure of voretigene neparvovec gene therapy is MLMT (Multi-Luminance Mobility Test) performance, which evaluates patients’ ability to navigate environments with variable illumination levels. In the presented group of four patients, a significant improvement in mobility was observed after therapy. All patients were able to move independently at significantly lower light intensity levels, indicating a significant improvement in vision under scotopic and mesopic conditions. Detailed MLMT results for this Polish patient cohort were published by our team separately in Klinika Oczna [Acta Ophthalmologica Polonica]. In the phase 3 study [8], patients treated with Luxturna were able to complete the obstacle course at lower illumination levels compared to untreated controls. Importantly, 65% of participants in the intervention group achieved the maximum possible level of improvement in the MLMT, passing the test at the lowest light intensity of 1 lux. None of the participants in the control group reached this level of improvement. Maguire et al. [9] analyzed the durability of treatment effects, including MLMT results, in patients who received Luxturna as part of phase 1 and phase 3 studies. The authors observed a consistent pattern of improvement in MLMT results across different patient groups, with maximum or near-maximum effects seen as early as day 30 post-treatment. Improvement compared to baseline was observed during the 1–4 year period after gene therapy.
In the presented case series, the surgical procedure of subretinal administration of voretigene neparvovec was completed without intraoperative complications in all four patients. It is worth emphasizing that the success of the subretinal procedure is crucial for the efficacy and safety of gene therapy. Improper surgical technique, incorrect cannula placement, or imprecise drug administration may lead to photoreceptor damage, subretinal hemorrhage, macular hole [8, 9], endophthalmitis [8], chorioretinal atrophy [6], or other complications, thereby reducing therapeutic effectiveness.
Several study limitations should be acknowledged here. First, the number of patients included in the analysis was small (n = 4), which is due to the rarity of the disease as well as the therapy’s highly specialized nature and high costs. While the results appear promising – with a particularly impressive visual parameter improvement in patient P3 – the limited sample size restricts the generalizability of the findings. Second, the mean follow-up period of 17.25 months (range: 3–42 months) was relatively short. In two patients (P3 and P4), the follow-up period was no longer than 6 months. Although previous clinical studies indicate that the effects of therapy persist for at least 4 years [12], the assessment of the long-term efficacy and safety of voretigene neparvovec requires a longer follow-up period. Third, the lack of a control group in this study limits the ability to definitively attribute the observed effects to the gene therapy alone. The natural course of the disease, as well as the placebo effect, can always influence the results obtained; however, in the case of a rare and progressive disease such as LCA, spontaneous improvement in vision is unlikely. A potential substitute for a control group is the fact that, due to economic constraints, only one eye per patient was treated. During the follow-up period, one patient (P1) experienced loss of light perception in the eye that did not receive the drug. In one case, the better-seeing eye was treated, whereas in three cases, the treated worse-seeing eye became the better-seeing eye by the end of the follow-up period. An additional limitation was the absence of retinal sensitivity measurements using Full-Field Light Sensitivity Testing (FST). Even though FST is a tool for assessing rod function and has been used in clinical studies of voretigene neparvovec [8], its use in the present patient group was not possible due to its unavailability in Poland. The FST test itself requires active cooperation and understanding of instructions by the patient, which can be difficult to achieve in younger patients. Nevertheless, the inability to conduct this test in our study does not compromise the overall evaluation of the therapy’s effectiveness. Importantly, the assessment of therapy efficacy was based on a comprehensive set of tests, including the MLMT. Furthermore, as described in the phase 3 clinical study for Luxturna, improvement in this test is strongly correlated with changes in FST [8].
In summary, the results of the present study involving Polish patients are consistent with previous clinical trial reports and confirm the therapeutic potential of voretigene neparvovec [8, 9, 12]. Our observations indicate that gene therapy represents a significant step toward improving the quality of life for patients with rare inherited retinal diseases. Starting in 2025, a drug program for Polish patients with retinal diseases caused by biallelic RPE65 gene mutations is set to become available. This program could significantly contribute to the wider implementation of this innovative treatment method in Poland. We anticipate that the continued development of novel gene therapies, along with advances in diagnostic and surgical techniques, will enhance prognosis and improve quality of life for a growing number of patients.

 POLSKI
POLSKI